查看更多
密码过期或已经不安全,请修改密码
修改密码
壹生身份认证协议书
同意
拒绝

同意
拒绝

同意
不同意并跳过





近几天来,大家都在疯传ICMJE的新规则:Data Sharing Statements for Clinical Trials — A Requirement of the International Committee of Medical Journal Editors。
我们主要从三个方面来讨论。最后讨论ICMJE的最新规则。
1、动物伦理和临床试验的伦理:课题设计完成,在开始实验/试验之前,一定要拿到动物伦理和临床试验的伦理。伦理问题,尤其是临床试验的伦理,是比较重要的,一定要有。当然,目前关于动物伦理,也已有SCI期刊要求提供。
请看我们投稿后,编辑部在2017年的回复邮件中提出的意见,是不是很“惊喜”:
Title: ***
Dear Professor ***,
Please provide the name of the committee from which you received ethical approval for the use of rats in your study, and provide evidence of this approval.
。。。
Yours sincerely,
***
2、临床试验注册:课题设计完成后,临床试验的课题,对于“临床试验注册”,大家也一定要注意,没有注册,估计后续会没有机会投稿。这个问题,似乎大家重视程度不够。
大家先请看要求提供临床试验注册的邮件(邮件是2015年编辑部回复的):
Dear Dr. **:
Before proceeding with the review orioles can you confirm the clinical trial registry number?(编辑部要求我们提供临床试验注册的注册号). I look forward to hearing from you.
Sincerely,
Dr. **
Journal of ***
大家再来看看编辑部因为作者没有按照ICMJE规则要求来注册,没有提前注册而是后补注册的后果(这个来自2016年编辑部的邮件):
Dear Dr. **,
Many thanks for clarifying the status of your clinical trial registration. Unfortunately, I cannot consider your manuscript further. As a journal, we adhere to the ICMJE guidelines, which require prospective trial registration(ICMJE的要求,前瞻性研究的临床试验,必须在纳入第一例病人前完成临床试验注册). In spite of the present decision, we look forward to submissions from you in the future.
Thank you for submitting your manuscript to the Journal of ***.
Yours sincerely
Dr. ***
然而,到这里,大家是不是认为:在还没有开始临床试验前,仅仅注册就可以了呢?看看下面最新的要求吧,目前的要求是越来越高!
3、ICMJE的新规:临床试验注册的数据,必须全部公开!!!这是最新的要求,就在6月初公布的。大家请看:

什么?什么?要全部公开?这是怎么回事??新规则下,医生还能不能好好发SCI论文了?
首先,我们看看ICMJE的要求是哪些?下图是ICMJE要求中提供的最新内容:
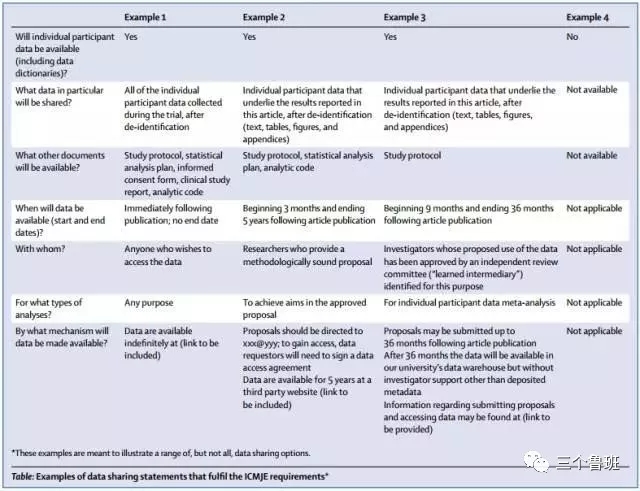
Therefore, ICMJE will require the following as conditions of consideration for publication of a clinical trial report in our member journals:
1. As of July 1, 2018, manuscripts submitted to ICMJE journals that report the results of clinical trials must contain a data sharing statement as described below.
2. Clinical trials that begin enrolling participants on or after January 1, 2019, must include a data sharing plan in the trial’s registration. The ICMJE’s policy regarding trial registration is explained at recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. If the data sharing plan changes after registration this should be reflected in the statement submitted and published with the manuscript and updated in the registry record.
简单翻译一下,就是:
第一,2018年7月及以后提交到ICMJE期刊的临床试验报告,必须包含数据共享声明。
第二,2019年1月1日后开始入组受试者的临床试验,必须在临床试验注册平台上提交数据共享计划。
ICMJE为何这么牛气?我们来看看ICMJE的情况,ICMJE是International Committee of Medical Journal Editors(国际医学期刊编辑委员会)的简称。
ICMJE的成员包括哪些期刊呢?医学领域的话,我们搜索了一下ICMJE的网站(),得到如下信息:
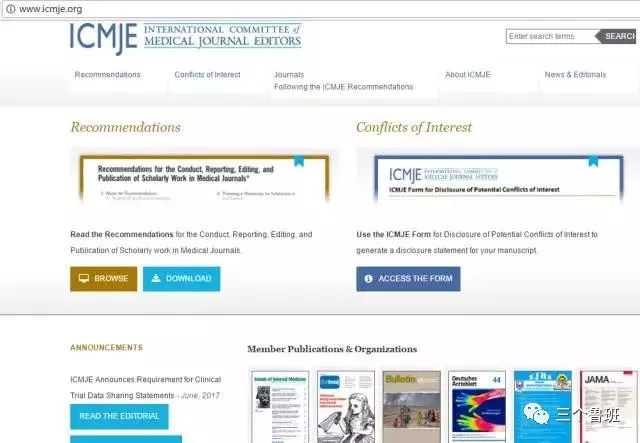
像什么NEJM、JAMA、Lancet、BMJ、Plos等都是ICMJE的成员。这个新的声明,估计后面会有全文的翻译版,目前在各大医学期刊上全文基本上都是免费下载的,大家可以自行前往查阅:
;
;
(17)31282-5/fulltext;
综上所述,后续大家在设计医学方面课题时,尤其是在实施前,各个规则都要考虑。不然,会给后面的发表带来不可控制的损失。新规则下,医生要想好好发SCI论文,就得遵守ICMJE的规则。
在投稿过程,编辑部返回的意见中,有的期刊在拒稿信中会写明需要遵守ICMJE和临床试验注册;而有的期刊只用一句话:不符合我们期刊刊文要求!草草打发掉,根本就不告诉我们到底是哪儿不符合,甚至发邮件咨询,编辑部还会说详情在for authors中。。。您老倒是告诉我们哪儿不行啊??
转载自:三个鲁班
查看更多